TECHNOLOGY: ICP Monitoring
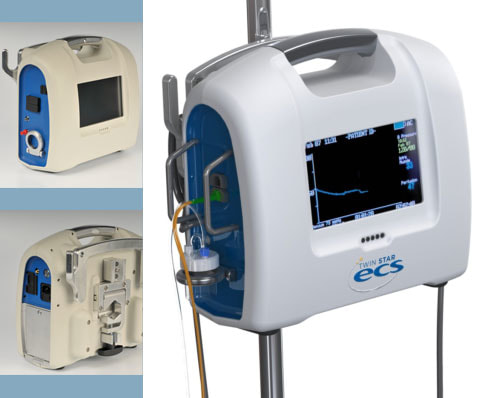
Client NeedA start-up medical device company developed a novel catheter for measuring pressure and draining excess fluid from the traumatized muscle tissue in order to treat compartment syndrome. The company required the custom electrical and software instrumentation needed to measure blood pressure, calculate/record/graph compartment pressure, supply periodic vacuum and function in a battlefield environment.
PROJECT SUMMARYWorking with the client’s industrial design group, Design Solutions designed and developed a custom integrated instrument that included an integrated TFT display with touch screen that could provide the necessary monitoring. Simple menu driven software allowed clinicians to monitor a patient while graphing and recording the pressure profiles with the data for each patient being achieved. A USB interface was provided to allow data transfer for further analysis. Additionally, considerable effort was taken to meet the military air worthy requirements for fixed and rotary winged aircraft that is required for patient transfer. Design Solutions designed and built eight pilot units and conducted verification testing, including Mil standards testing. The device is currently undergoing military qualification.
|
skills applied
|
a collaborative team partnership
We take pride in our extensive range of services to our clients. Our ISO 13485:2016 certified engineering design process assures our customers
that we can fulfill their needs at every level of the medical device design and development process.
that we can fulfill their needs at every level of the medical device design and development process.