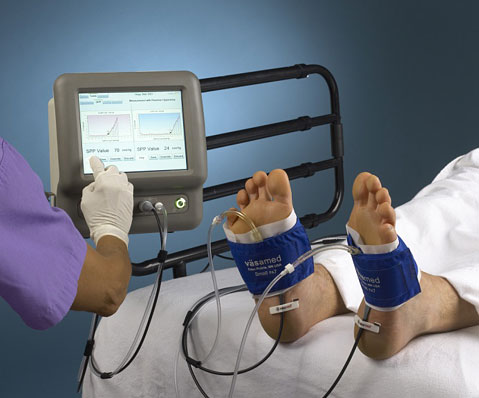
Client NeedA customer required an update to their current product line of peripheral artery disease detection devices. The current device was on a cart system and measured only a single channel with the instrumentation and laptop combination. The customer determined there was a market need for a single enclosure system that was capable of measuring two channels and was able to mount on a pole or bedside.
PROJECT SUMMARYDesign Solutions was contracted to provide the electrical design and system configuration working with an outside industrial design firm. A key requirement of the design was to allow porting of the software code base from the current laptop based unit into the new design. We worked with the customer’s software engineers to ensure that the technology chosen was compatible with the legacy code base. The project was completed and Design Solutions worked with the client’s regulatory group in order to obtain the necessary approvals for safety. The product is currently being marketed and sold in the United States and Japan.
|
skills applied
|
a collaborative team partnership
We take pride in our range of services to our clients. Our ISO 13485:2016 certified design process assures our customers that we can fulfill their needs at every level of the medical device design process.